Inhaled product expertise

Expertise in inhaled product & pharmaceutical development
INHALEXPERT’s offer is based on a broad expertise and high skills in inhaled product development.
Expertise based on 25 years’ experience in pharmaceutical development, including 16 years in GlaxoSmithKline, worldwide leader in respiratory field.
More than a dozen products studied including development of Nasal spray, Metered Dose Inhaler (MDI) and Dry Powder Inhaler (DPI) with the Seretide Diskus, 2nd worldwide drug product, leader in asthma and COPD treatment.
This specialist skill combines with strong experience in project management allows INHALEXPERT to design, structure and manage all the product development and to bring expertise in inhaled product development.
From consulting to the lead of inhaled product development, Inhalexpert is your partner.
Inhalexpert activities encompasses
- Pharmaceutical development strategy and tactics
- Preparation of development plans, project management, management of CRO’s
- Device design and development of inhalation system adapted to the strategy in line with the targeted therapeutic needs
- Pharmaceutical development (Formulation, micronisation/process, analytic and physical properties activities)
- Coupling product formulation and device
- Industrialization and launch
- Critical aspect of regulatory CMC dossier
Developing with Inhalexpert a new inhaled product
- Offers new opportunities and alternatives to molecules, R&D valued and profitable
- Creates intellectual property and a product hard to copy
- Opens access to new market share
One of the oldest route
potentially suitable for the delivery of a wide range of drugs
History
In India 4000 years ago, leaves of the Atropa Belladonna plant, containing atropine as bronchodilator, were smoked as a cough suppressant.
Modern inhalation therapy began in the nineteenth-century with the invention of the glass bulb nebulizer.
First patented DPI (granted in 1852), no evidence of commercial success.
Over the past decades inhalation therapy has established itself as a valuable tool in the local therapy of pulmonary diseases, such as asthma or COPD.
The development of the first p-MDI for asthma therapy in 1956 was a major advance. However, the required hand-lung coordination of the patient and the use of environmentally damaging CFC propellants, are major drawbacks of the traditional p-MDI.
Dry powder inhalers (DPI’s) were introduced to overcome these drawbacks. From the first introduction of a DPI in 1971 (Spinhaler), several DPI’s have been added to the collection of available inhalers. They are breath-controlled and so coordination problems have been overcome.
DPI’s are also potentially suitable for the delivery of a wide range of drugs, such as anti-asthmatics, peptides and proteins. DPI’s can also deliver a wide range of doses from 5 μg to more than 20 mg via one short inhalation.
Inhaled route
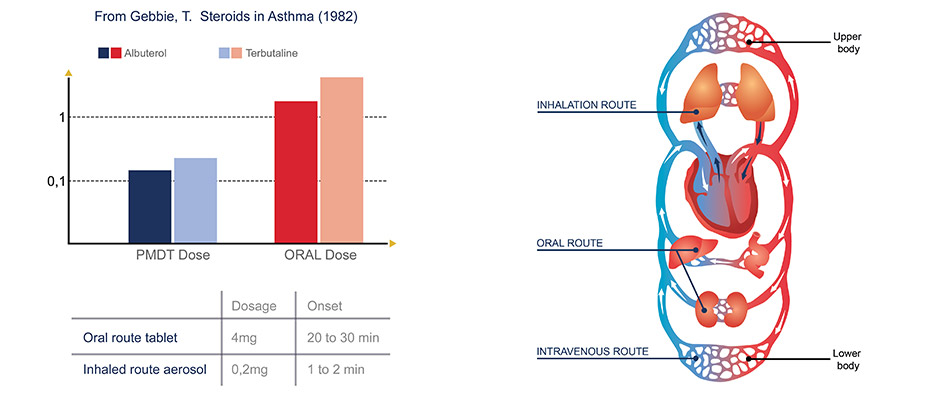
Inhaled route benefits
Systemic Effect
Absorption area of about 100m2 very permeable with important vascularization:
- Homogeneous infused surface
- 300-500Mi of alveoli
- Thin barrier cell / capillary: 2.2 microns
- Systemic absorption higher than by oral route
- Minimize the dose required,
- Minimize side effects,
- Reduction of API cost
No degradation due to gastrointestinal tract passage
Avoid hepatic first-pass metabolism
Non-invasive, improved patient comfort
Local effect
- Minimize the dose required
- Minimize side effects
- Reduction of API cost
No degradation due to gastrointestinal tract passage
Avoid hepatic first-pass metabolism
Non-invasive, improved patient comfort
COPD is the fourth worldwide leading cause of death
There are 300 million people with asthma
Inhaled route has long been favored for treatment of respiratory diseases.
Moreover, asthma and COPD major treatments will lose their patents in the years to come.
Mastering the development of inhaled products may represent an opportunity for the development of new treatment and to develop generic products.
“The respiratory market traditionally represents one of the most significant sources of revenue for the branded pharmaceutical industry, with high barriers to entry for new companies and generics manufacturers”.
Business insights: The Asthma, COPD & Allergic Rhinitis Market Outlook to 2011
Fields of application
Few examples of potential or existing pulmonary local treatment-no
Flu Antiviral: zanamivir
Mucoactive drug: Nacystelyn
Cystic fibrosis (CF): Tobramycin inhalation solution (antibiotic) to treat P. aeruginosa. Recombinant human DNase
HIV-infected (AIDS) or immunocompromised patients: Inhaled pentamidine solution = anti-infective agent
Potential or existing treatment using inhaled route for systemic effect-no
Migraine: Ergotamine tartrate
Cancer pain: Fentanyl
Analgesia: Morphine sulfate
Parkinson desease: Apomorphine hydrochloride for the treatment of off-periods in Parkinson’s disease not responding to oral treatment
Pulmonary arterial hypertenstion: Inhaled iloprost, analog of prostacyclin (Ventavis1) with the I-neb1 AAD
Diabetes: Insulin (Exubera)
Vaccines: influenza, BCG, measles
Sexual dysfunction: Sildenafil
Otitis media: Augmentin
Osteoporosis: Calcitonine
Anticoagulant activity: Heparin

