Quality by Design Support

“Quality cannot be tested into products; it should be built-in or should be by design.”
“By beginning with the end in mind, the result of development is a robust formulation and manufacturing process with an acceptable control strategy that ensure the performance of the drug product“
Quality by Design is highly recommended by ICH through ICH Q8, ICH Q9, ICH Q10 and ICH Q11. Comprehensive implementation of the three guidelines i.e. ICH Q8, Q9 and Q10 together is essential to achieve ICH Quality Vision.
Inhalexpert has built its expertise and experience through QbD projects during more than 10 years. Expertise and experience completed through support of branded, bio and generic companies. Inhalexpert is also QbD trainer at Paris and Angers Faculties of Pharmacy – FRANCE.
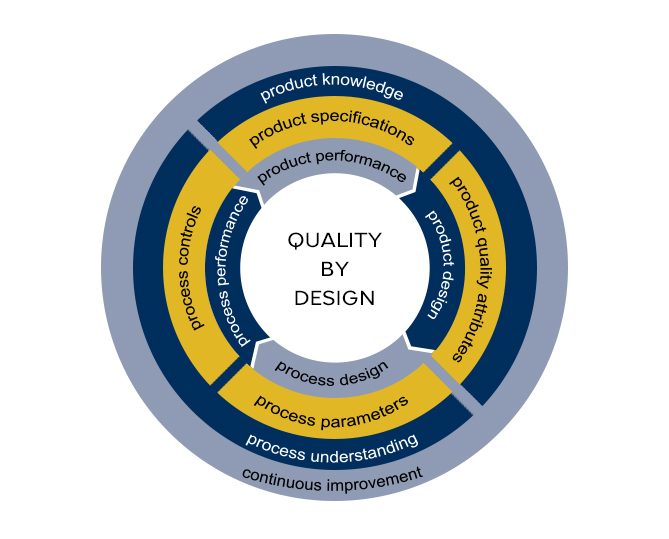
QbD expertise: pathway to the desired state
INHALEXPERT
- provides its expertise and experience in QbD concept and pragmatic approach including QbD tools,
- provides training and project management support according to your need,
- adapts the concept to your development i.e. branded, bio and generic drug product,
- assists companies to evolve structures, ways of working, coordination, communication and management,
- integrates objectives and constraints, works together with its customers to improve processes, methods and results.

